Affiliate links on Android Authority may earn us a commission. Learn more.
What's the difference between a Li-ion and solid-state battery?

A couple of weeks ago, Kris introduced us to the topic of solid-state batteries and how they might be the next major advancement in smartphone battery technology. In short, solid-state batteries are safer, can pack in more juice, and can be used for even thinner devices. Unfortunately, they’re prohibitively expensive to put into medium-sized smartphone cells right now, but that might change in the coming years.
So, if you’ve been wondering what exactly a solid-state battery is and how it’s different to today’s lithium-ion cells, read on.

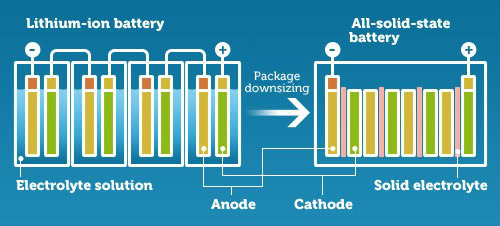
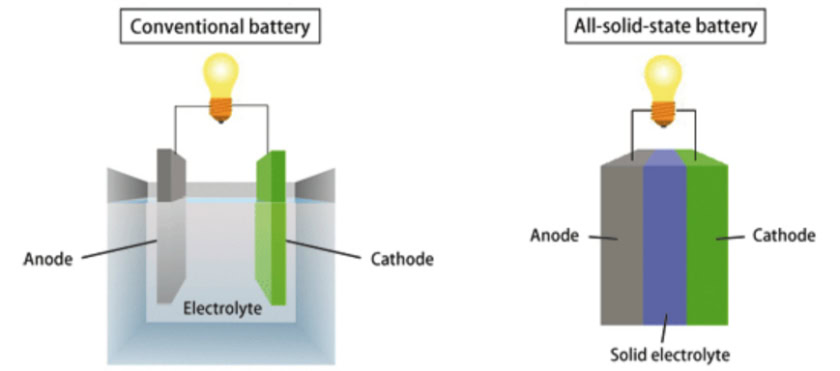
The key difference between the commonly used lithium-ion battery and a solid-state battery is that the former uses a liquid electrolytic solution to regulate the flow of current, while solid-state batteries opt for a solid electrolyte. A battery’s electrolyte is a conductive chemical mixture that allows the flow of current between the anode and cathode.
Solid state batteries still work in the same way as current batteries do, but the change in materials alters some of the battery’s attributes, including maximum storage capacity, charging times, size, and safety.

Space saving
The immediate benefit of switching from a liquid to solid electrolyte is that the energy density of the battery can increase. This is because instead of requiring large separators between the liquid cells, solid state batteries only require very thin barriers to prevent a short circuit.
Solid-state batteries can pack in twice as much energy as Li-ion
Conventional liquid-soaked battery separators come in with a 20-30 micron thickness. Solid-state technology can decrease the separators down to 3-4 microns each, a roughly 7-fold space saving just by switching materials.
However, these separators aren’t the only component inside the battery and other bits can’t shrink down as much, putting a limit on the space-saving potential of solid-state batteries.
Even so, solid-state batteries can pack in up to twice as much energy as Li-ion, when replacing the anode with a smaller alternative as well.
Longer life spans
Solid-state electrolytes are typically less reactive than today’s liquid or gel, so they can be expected to last a lot longer and won’t need replacing after just 2 or 3 years. This also means that these batteries won’t explode or catch fire if they are damaged or suffer from manufacturing defects, meaning safer products for consumers.
Solid-state batteries won't explode or catch fire if they are damaged or suffer from manufacturing defects.
In current smartphones, replaceable batteries are often sought after for those looking to use the same phone for many years, as they can be swapped out once they start to break down.
Smartphone batteries often don’t hold their charge as well after a year or so and can even cause hardware to become unstable, reset, or even stop working after several years of use. With solid-state batteries, smartphones and other gadgets could last a lot longer without needing a replacement cell.
There are plenty of solid chemical compounds that could be used in batteries, not just one.
Talk of liquid versus solid batteries is an oversimplification of the subject though, as there are plenty of solid chemical compounds that could be used in batteries, not just one.
Types of solid-state electrolytes
There are eight different major categories of solid-state batteries, which each use different materials for the electrolyte. These are Li-Halide, Perovskite, Li-Hydride, NASICON-like, Garnet, Argyrodite, LiPON, and LISICON-like.
As we’re still dealing with an emerging technology, researchers are still coming to grips with the best types of solid-state electrolyte to use for different product categories. None have come out as clear leaders just yet, but sulfide-based, LiPON, and Garnet cells are currently seen as the most promising.
You’ll probably have noticed that many of these types are still lithium (Li) based in some regard, because they are still using lithium electrodes. But many are opting for new anode and cathode electrode materials to improve performance.
Thin film batteries
Even within solid-state battery types, there are two clear cut subtypes – thin film and bulk. One of the most successful thin-film types that is already on the market is LiPON, which the majority of manufacturers produce with a lithium anode.
The LiPON electrolyte offers excellent weight, thickness, and even flexibility attributes, making it a promising cell type for wearable electronics and gadgets that require small cells. Going back to the subject of longer lasting cells, LiPON has also demonstrated excellent stability with only a 5% capacity reduction after 40,000 charge cycles.
LiPON batteries could last anywhere from 40 to 130 times longer than Li-ion batteries before they need replacing.
For comparison, lithium-ion batteries only offer between 300 and 1000 cycles before showing a similar or greater fall in capacity. This means that LiPON batteries could last anywhere from 40 to 130 times longer than Li-ion batteries before they need replacing.
LiPON’s downside is that its total energy storage capacity and conductivity are rather poor by comparison. However, alternative solid-state battery technologies could be the key to bringing longer battery life to smartwatches, which is currently putting off a number of customers from picking up a wearable.
Bigger, bulkier batteries
So far, solid state batteries aren’t yet suitable for larger cells found in smartphones and tablets, let alone laptops or electric cars. For larger bulk solid-state batteries with a greater capacity, superior conductivity that comes close to or matches liquid electrolytes is required, which rules out otherwise promising technologies like LiPON. Ionic conduction measures the ability of ions to move through a material, and good conduction is a requirement of larger cells to ensure the required current.
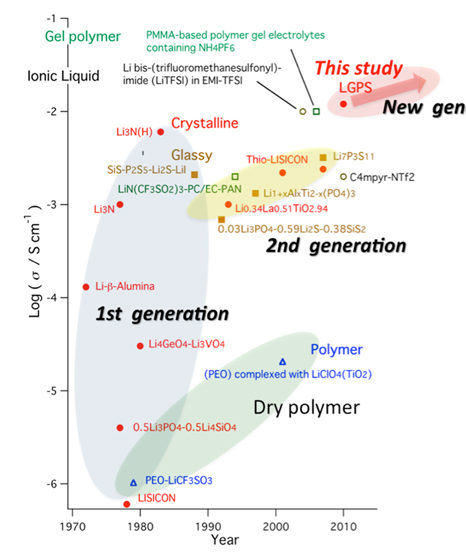
LISICON and LiPS have overtaken research into LiPO, LiS, and SiS batteries, the previous leaders in the solid state field. However, these types still suffer from lower conductivity than organic and liquid electrolytes at room temperature, making them impractical for commercial products.
Highly conductive
This is where research into garnet-oxide (LLZO) electrolytes comes in, as it boasts a high ionic conductivity at room temperature.
The material achieves a conduction that comes in only slightly behind the results offered by liquid lithium-ion cells, and new studies into LGPS suggest that this material could even match it.
This would mean solid-state batteries of roughly equal power and capacity as today’s Li-ion cells, while seeing benefits such as reduced size and longer lifespan become a reality.
Garnet is also stable in air and water, making it suitable for Li-Air batteries too. Unfortunately it has to be fabricated using an expensive sintering process.
This currently makes it an unattractive proposition for use in consumer batteries when compared to the low cost of lithium-ion cells. In the future, costs are likely to fall as manufacturing techniques are refined but we are still some way off from a commercially viable solid-state battery.

Wrap up
Clearly there is still a lot of ongoing research into solid-state battery technology. We’re not going to see mature cells make their way into consumer products like smartphones for another 4 or 5 years, according to the earliest predictions. Solid-state batteries in other devices (like drones) may appear as soon as next year though.
Still, the latest research is finally producing results that can compete with existing li-ion batteries in terms of attributes, while also providing the benefits of solid-state electrolytes. All we need is for manufacturing processes to mature, and there are a number of large and upcoming battery manufacturers with the resources to make this a reality.
In summary, the key benefits of all these chemical differences from a consumer perspective are: up to 6 times faster charging, up to twice the energy density, a longer cycle life of up to 10 years compared to 2, and no flammable components. That’s certainly going to be a boon for smartphones and other portable gadgets.