Affiliate links on Android Authority may earn us a commission. Learn more.
Vanadium dioxide is a strange metal that doesn’t heat up while conducting electricity
A study led by scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and at the University of California, Berkeley, has just made a groundbreaking discovery which could have major implications not just for future consumer gadgets, but for a range of other engineering disciplines. The findings reveal that vanadium dioxide (VO2) nanobeams can conduct electricity without conducting heat, meaning that it breaks the Wiedemann-Franz Law.
Whether you’re an experienced engineer or hobbyist student of electronics, you’ll probably be aware that materials that are good conductors of electricity are also good conductors of heat. This is known as the Wiedemann-Franz Law, a theory that applies to all metallic materials. However, vanadium dioxide breaks this rule.
The scientists discovered that vanadium dioxide’s thermal conductivity attributed to the electrons is ten times smaller than what would be expected from the Wiedemann-Franz Law. This strange effect is due to the way that electrons move inside the material, moving in unison like a fluid rather than acting as individual particles as they do in regular metals. This “coordinated marching-band-like motion” prevents heat transfer from occurring as efficiently as in usual metals, but doesn’t impede the movement of electrons.
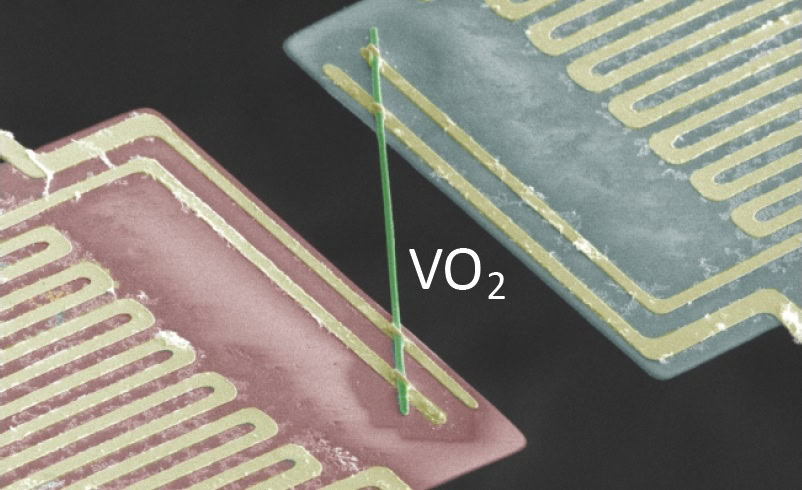
Another very interesting feature of VO2 is that its electrical and heat conductivity can be switched on and off at different temperatures. This is known as a phase transition and the material can either be metallic (conductive) or an insulator. The researchers were able to adjust both the heat and electronic conductivity properties by mixing VO2 with other compounds, as well as adjusting the phase transition temperature.
Interestingly, scientists were able to lower the temperature threshold for vanadium dioxide’s electric conductivity by mixing it with other materials, like metal tungsten. Mixing also encouraged the vanadium dioxide’s electrons to carry heat more effectively in the metallic phase, and it seems that the researchers are able to tune the amount of conduction for both phases, meaning that the material can be manipulated for a lot of different purposes.
Vanadium dioxide has also previously been tipped as a potentially useful material to help build “super field effect transistors” or Mott insulators, due to its switchable metallic and insulating properties at different temperatures. VO2 transistors are theorised to be able to improve a transistor’s ratio of on current to off current.
This is because phase transition occurs through the entire VO2 material, whereas existing FET conductivity is influenced by only a thin layer close to where the current flows between gate and drain, which reduces the effectiveness of the switching ratio. What this means is that transistors could reach their peak current faster, reducing the amount of current wasted as heat and improving their switching speed, which could be used to produce faster and cooler processors.
“[This research] shows a drastic breakdown of a textbook law that has been known to be robust for conventional conductors. This discovery is of fundamental importance for understanding the basic electronic behavior of novel conductors.” – Junqiao Wu, a physicist at Berkeley Lab’s Materials Sciences Division and a UC Berkeley professor of materials science and engineering.
As well as electronics applications, the research team suggests that the material could be used to help stabilize temperatures in a variety of situations, as the material can dissipate more heat in the summer and prevent heat loss in the winter, as its conductivity changes with temperature.
Vanadium dioxide is also tipped to be useful in thermoelectric systems that convert waste heat from engines and appliances into electricity. This could mean that wasted heat and energy from your phone’s battery, processor, etc could be converted back into usable power using this material, increasing battery life.
Vanadium dioxide’s properties certainly make it a promising material for a variety of applications, and we may even see it used in mobile applications in the future. For now, the researchers note that there are more questions that need to be answered before vanadium dioxide can be commercialized. In other words, there’s lots of potential but we likely won’t be seeing it commercially for quite some time.